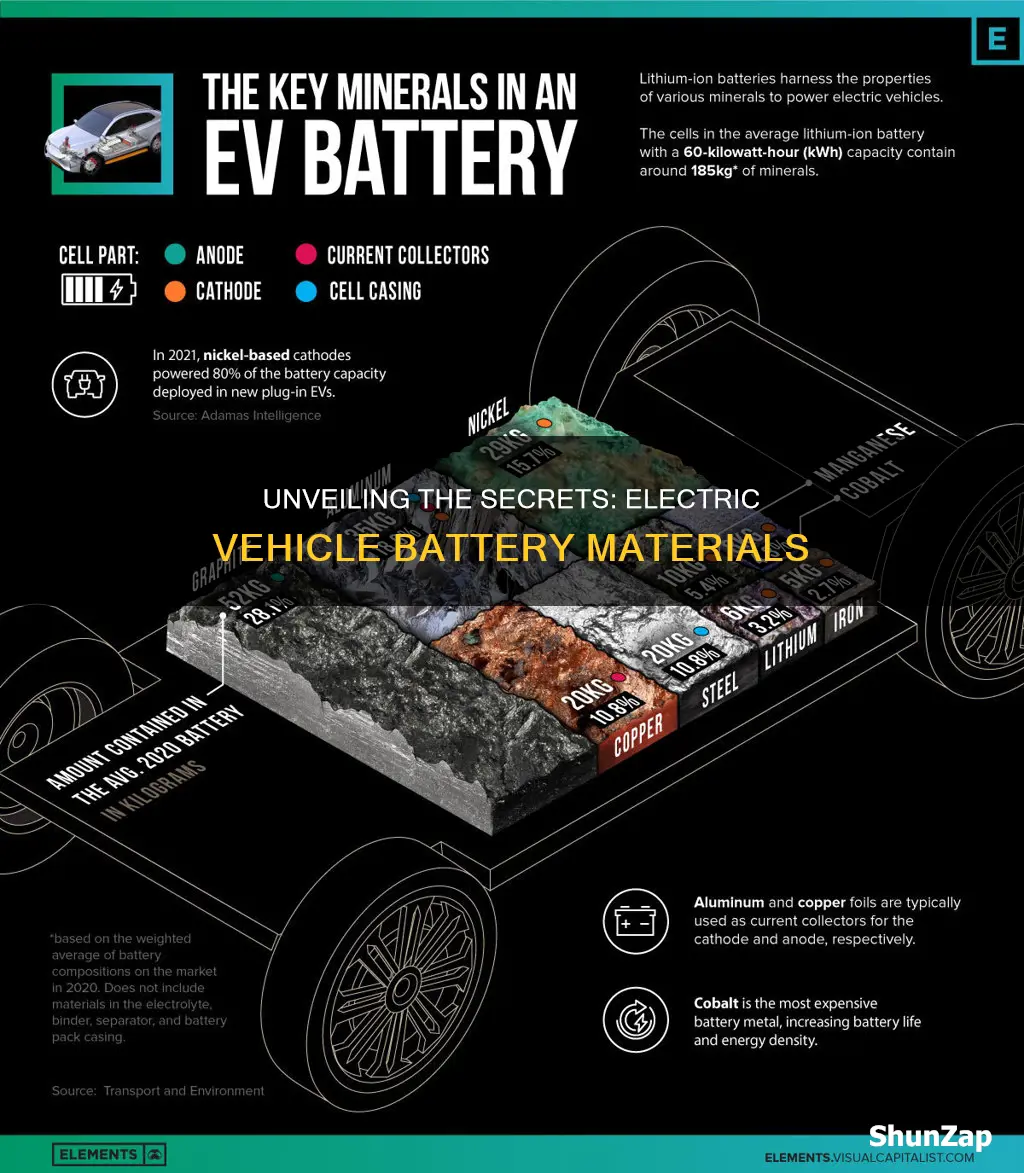
Electric vehicle batteries, the heart of these sustainable vehicles, are composed of various materials, each playing a crucial role in their functionality and performance. These batteries are typically made from a combination of lithium, cobalt, nickel, manganese, and other elements, depending on the specific battery chemistry. The positive electrode, or cathode, often contains lithium-based compounds like lithium cobalt oxide (LiCoO2), while the negative electrode, or anode, is usually made of graphite. The electrolyte, a crucial component, facilitates the movement of ions between the electrodes, enabling the battery to store and release energy. Understanding the composition of these batteries is essential for optimizing their performance, safety, and environmental impact.
What You'll Learn
- Lithium-ion Chemistry: Anode, cathode, and electrolyte materials
- Nickel-based Alloys: Used in high-performance batteries
- Graphene: A promising material for battery electrodes
- Solid-state Electrolytes: Potential replacement for liquid electrolytes
- Recycling and Sustainability: Focus on eco-friendly battery production

Lithium-ion Chemistry: Anode, cathode, and electrolyte materials
The lithium-ion battery, a cornerstone of electric vehicles (EVs), operates on the principles of lithium-ion chemistry, where lithium ions move between the anode and cathode during charging and discharging. This movement is facilitated by the electrolyte, a critical component that enables the flow of ions while preventing electrical contact between the electrodes.
Anode Materials:
The anode, typically made of graphite, is a crucial component in lithium-ion batteries. Graphite's layered structure allows lithium ions to intercalate (insert) between its layers during charging, expanding the anode's volume. This process is reversible, enabling the battery to release stored energy during discharge. The specific lithium compounds used in the anode can vary, with common choices including lithium cobalt oxide (LCO) and lithium iron phosphate (LFP). LCO, a spinel structure, offers high energy density but may suffer from thermal stability issues. LFP, on the other hand, provides a safer alternative with improved thermal stability, making it a popular choice for EV batteries.
Cathode Materials:
Cathode materials play a vital role in determining the battery's performance and safety. Common cathode materials include lithium nickel manganese cobalt oxide (NMC) and lithium iron phosphate (LFP). NMC, with its layered structure, offers high energy density and is widely used in EV batteries. However, it can pose safety risks due to its potential for thermal runaway. LFP, another popular choice, provides a safer alternative with improved thermal stability, making it suitable for EV applications.
Electrolyte Materials:
The electrolyte is a critical component that facilitates the movement of lithium ions between the anode and cathode. It is typically a lithium salt dissolved in a solvent, such as ethylene carbonate (EC) or diethyl carbonate (DEC). The choice of solvent and salt significantly impacts the battery's performance and safety. For instance, EC, known for its high thermal stability, is often used in high-temperature batteries, while DEC offers better low-temperature performance. The electrolyte's viscosity and conductivity are also essential factors, influencing the battery's overall efficiency and cycle life.
In summary, the lithium-ion battery's performance and safety are closely tied to the choice of anode, cathode, and electrolyte materials. Graphite, NMC, and LFP are commonly used in these applications, each offering unique advantages and trade-offs. The development of advanced materials continues to drive improvements in energy density, safety, and overall battery performance, making lithium-ion technology a key enabler for the widespread adoption of electric vehicles.
Upgrading Your Ride: A Guide to Converting Vehicle Axles to Electric Brakes
You may want to see also

Nickel-based Alloys: Used in high-performance batteries
Nickel-based alloys are an essential component in the development of high-performance batteries for electric vehicles (EVs). These alloys, primarily composed of nickel, offer a range of benefits that make them ideal for use in advanced battery systems. One of the key advantages of nickel-based alloys is their ability to provide high energy density. This is crucial for EVs, as it allows for a more compact and lightweight battery design, enabling longer driving ranges without compromising on performance. The high energy density of nickel-based alloys is achieved through their unique crystal structure, which allows for efficient storage and release of energy.
In the context of EV batteries, nickel-based alloys are often used in the cathode material. The cathode is responsible for storing and releasing lithium ions during the charging and discharging cycles of the battery. Nickel-based cathodes, such as nickel-cobalt-manganese (NCM) and nickel-cobalt-aluminum (NCA) compounds, offer improved thermal stability and cycle life compared to traditional cathodes. This enhanced stability is particularly important for high-performance batteries, as it ensures consistent performance even under extreme conditions, such as rapid charging and discharging.
The use of nickel-based alloys in EV batteries also contributes to their overall safety. These alloys exhibit excellent thermal stability, reducing the risk of thermal runaway, a potentially dangerous situation where the battery overheats and releases flammable gases. By minimizing the chances of thermal issues, nickel-based alloys help to ensure the safe operation of high-performance batteries. Additionally, the stability of these alloys allows for a longer lifespan, as they can withstand numerous charge-discharge cycles without significant degradation.
Furthermore, nickel-based alloys offer a good balance of electrical conductivity and mechanical strength. This combination is vital for efficient energy transfer and structural integrity within the battery. The alloys' conductivity ensures rapid charging and discharging, while their strength provides structural support, preventing degradation and maintaining the battery's overall performance over time.
In summary, nickel-based alloys play a critical role in the development of high-performance batteries for electric vehicles. Their high energy density, thermal stability, safety features, and balanced electrical and mechanical properties make them an ideal choice for advanced battery systems. As the demand for more efficient and sustainable transportation continues to grow, the use of nickel-based alloys in EV batteries will likely become even more prevalent, contributing to the advancement of the electric vehicle industry.
Nissan Kicks: Electric Vehicle or Not? Unveiling the Truth
You may want to see also

Graphene: A promising material for battery electrodes
Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, has emerged as a highly promising material for battery electrodes, particularly in the context of electric vehicles (EVs). Its unique properties make it an ideal candidate to enhance battery performance and address some of the challenges associated with traditional battery materials.
One of the key advantages of graphene is its exceptional electrical conductivity. Graphene's high electron mobility allows for rapid electron transfer, which is crucial for efficient charge and discharge processes in batteries. This property can lead to faster charging times and improved overall battery efficiency. Additionally, graphene's excellent thermal conductivity helps dissipate heat, a critical factor in managing battery temperature during operation, especially in high-power applications like EVs.
In the context of battery electrodes, graphene's structural integrity is another significant benefit. Its two-dimensional nature provides a large surface area, which is essential for maximizing the number of active sites available for electrochemical reactions. This increased surface area can lead to higher energy density and improved capacity, allowing batteries to store more energy in a given volume. Furthermore, graphene's flexibility and strength make it suitable for use in flexible and bendable battery designs, a feature that could be valuable for the development of innovative battery shapes and forms.
The use of graphene in battery electrodes also offers the potential for improved cycle life and reduced degradation. Graphene's stability under repeated charge-discharge cycles can lead to longer-lasting batteries with minimal capacity loss over time. This is particularly important for EVs, where battery longevity and reliability are essential for consumer acceptance and widespread adoption.
However, there are challenges to be addressed. One issue is the large-scale production of high-quality graphene at a cost-effective price. While graphene has shown great promise in laboratory settings, scaling up production to meet the demands of the EV market requires further research and development. Additionally, ensuring the compatibility of graphene with existing battery architectures and materials is crucial for its successful integration into commercial battery systems.
In summary, graphene's unique properties, including its electrical conductivity, thermal management capabilities, and structural integrity, make it a highly promising material for battery electrodes in electric vehicles. Ongoing research aims to overcome production and compatibility challenges, with the ultimate goal of harnessing graphene's potential to revolutionize battery technology and contribute to the widespread adoption of electric mobility.
Chevy Trax: Electric Vehicle or Not? Unveiling the Truth
You may want to see also

Solid-state Electrolytes: Potential replacement for liquid electrolytes
The quest for improved battery technology in electric vehicles (EVs) has led to significant research and development efforts, with one of the most promising areas being the replacement of traditional liquid electrolytes with solid-state electrolytes. This shift is driven by the potential to enhance battery performance, safety, and longevity. Solid-state electrolytes, as the name suggests, are materials that conduct electricity in a solid state, offering a range of advantages over their liquid counterparts.
One of the key benefits of solid-state electrolytes is their ability to address the safety concerns associated with liquid electrolytes, particularly in the context of thermal runaway. Liquid electrolytes, such as those containing flammable organic solvents, can pose risks due to their volatility. In contrast, solid-state electrolytes are inherently safer as they do not contain volatile components that can ignite or explode. This makes them an attractive option for EV manufacturers aiming to improve the overall safety of their battery systems.
Additionally, solid-state electrolytes can contribute to higher energy density, a critical factor for extending the range of electric vehicles. These electrolytes can facilitate faster ion transport, enabling the development of batteries with higher power and energy density. This is particularly important for EV manufacturers who are constantly seeking ways to increase the driving range of their vehicles without significantly increasing their size or weight.
The development of solid-state electrolytes has been a focus of research for many years, with various materials being explored. One of the most promising candidates is a type of solid polymer electrolyte, which can provide excellent thermal stability and high ionic conductivity. These polymers are designed to be flexible and durable, allowing for their integration into various battery designs. Another approach involves using ceramic materials, which offer high temperature stability and excellent chemical resistance, making them suitable for high-performance batteries.
Furthermore, solid-state electrolytes can contribute to the reduction of battery weight and volume. As these electrolytes eliminate the need for bulky liquid containers, they can lead to more compact and lightweight battery designs. This is a significant advantage for EV manufacturers, as it allows for more efficient use of space and potentially reduces the overall weight of the vehicle, thereby improving its performance and efficiency.
In summary, solid-state electrolytes present a compelling solution for the future of electric vehicle batteries. Their ability to enhance safety, increase energy density, and contribute to more compact designs makes them a key area of focus for researchers and manufacturers alike. As the development of these technologies continues, we can expect to see significant advancements in EV battery performance, paving the way for a more sustainable and efficient transportation future.
Strategies for Accelerating the Shift to Electric Vehicles
You may want to see also

Recycling and Sustainability: Focus on eco-friendly battery production
The growing popularity of electric vehicles (EVs) has sparked a crucial conversation about the environmental impact of their batteries. As the demand for sustainable solutions increases, it is essential to explore eco-friendly battery production methods and the recycling processes that can extend the lifespan of these batteries.
Electric vehicle batteries are primarily composed of lithium-ion technology, which has become the standard in the industry. These batteries are made from a combination of materials, including lithium, cobalt, nickel, manganese, and graphite. The positive electrode (cathode) is typically made of a lithium-based compound, such as lithium cobalt oxide (LiCoO2), while the negative electrode (anode) is often constructed from graphite. This design allows for efficient energy storage and release, making it ideal for powering EVs. However, the extraction and processing of these materials can have significant environmental consequences.
To address these concerns, researchers and engineers are focusing on developing sustainable practices. One approach is to improve the recycling processes of EV batteries. Recycling lithium-ion batteries is crucial to reducing the demand for raw materials and minimizing the environmental impact of mining. The recycling process involves several steps: first, the batteries are disassembled, and the cathode and anode materials are separated. The cathode can be recycled to produce new battery components, while the anode graphite can be processed to create new anodes or other materials. This recycling process not only reduces waste but also ensures a more sustainable supply chain for the EV industry.
Additionally, efforts are being made to develop eco-friendly battery chemistries. Scientists are exploring alternative materials that are more abundant and environmentally friendly. For example, solid-state batteries use a solid electrolyte instead of a liquid one, potentially reducing the risk of fire and improving safety. Another approach is to incorporate more sustainable metals like zinc and aluminum into battery designs, which could decrease the reliance on rare earth metals. These innovations aim to create batteries that are not only powerful and efficient but also environmentally conscious.
Furthermore, the concept of a circular economy is being applied to battery production. This involves designing batteries with end-of-life recycling in mind, ensuring that materials can be reused or safely disposed of. Manufacturers are also working on developing more efficient manufacturing processes to reduce energy consumption and waste generation during production. By adopting these sustainable practices, the EV battery industry can contribute to a greener future, minimizing the environmental footprint of electric vehicles.
Cummins' Electric Future: Powering a Sustainable Revolution
You may want to see also
Frequently asked questions
Electric vehicle batteries primarily use lithium-ion technology, which relies on a combination of lithium, cobalt, nickel, manganese, and other elements. These materials are carefully selected for their ability to store and release energy efficiently.
Lithium is a key component due to its high electrochemical potential, allowing it to provide a high energy density. It enables the battery to store a significant amount of energy in a relatively small space, making it ideal for powering electric vehicles.
Yes, researchers are actively investigating various alternatives to traditional lithium-ion batteries. Some focus on solid-state batteries using solid electrolytes, while others explore sodium-ion or potassium-ion technologies. These alternatives aim to improve energy density, safety, and sustainability compared to conventional lithium-ion batteries.